
The global next-generation biomanufacturing market size was valued at USD 26.61 billion in 2024 and is expected to reach USD 56.43 billion by 2032, at a CAGR of 9.85% during the forecast period
IntroductionThe Next-Generation Biomanufacturing Market represents the global industry focused on advanced biological production systems used to manufacture biologics, vaccines, cell and gene therapies, enzymes, and bio-based products. This market integrates innovative technologies such as single-use systems, continuous bioprocessing, synthetic biology, and digital manufacturing platforms to improve efficiency, scalability, and sustainability.
The global importance of next-generation biomanufactauring continues to expand due to rising demand for biologics, personalized medicine, and sustainable production methods. Governments, pharmaceutical companies, and biotechnology firms rely on advanced biomanufacturing to ensure secure supply chains, rapid product development, and cost-effective production.
Learn how the Next-Generation Biomanufacturing Market is evolving—insights, trends, and opportunities await. Download report: https://www.databridgemarketresearch.com/reports/global-next-generation-biomanufacturing-market
The Evolution
The evolution of biomanufacturing began with traditional batch-based fermentation and cell culture systems used primarily for antibiotics and basic biologics. Early production models relied on large stainless-steel bioreactors, manual processes, and long development timelines.
Key milestones emerged with the commercialization of recombinant DNA technology, enabling large-scale production of insulin, growth hormones, and monoclonal antibodies. This period marked the foundation of modern biopharmaceutical manufacturing.
The transition toward next-generation biomanufacturing accelerated in the early 2000s with the adoption of single-use bioreactors, modular facilities, and process analytical technologies. These innovations reduced contamination risks, lowered capital costs, and increased operational flexibility.
Recent shifts in demand focus on speed, scalability, and precision. The rise of cell and gene therapies, mRNA vaccines, and personalized biologics has driven the need for continuous processing, automation, and advanced digital control systems. Biomanufacturing now emphasizes adaptability to small-batch, high-value products alongside large-scale production.
Market Trends
The next-generation biomanufacturing market is shaped by strong technological and operational trends. One prominent trend is the adoption of continuous bioprocessing, which improves productivity, consistency, and resource utilization compared to traditional batch processes.
Automation and digitalization play a central role. Manufacturers increasingly deploy artificial intelligence, machine learning, and digital twins to optimize process control, reduce downtime, and improve yield predictability. Real-time monitoring enhances quality assurance and regulatory compliance.
Sustainability influences production strategies. Companies focus on reducing water usage, energy consumption, and waste generation through process intensification and bio-based raw materials. Circular bioeconomy principles gain importance across industrial biomanufacturing.
Regionally, North America leads in technology adoption due to strong biopharmaceutical R&D investment and manufacturing capacity. Europe emphasizes sustainable biomanufacturing and regulatory harmonization. Asia-Pacific shows rapid growth driven by expanding biopharma production, government incentives, and growing domestic demand.
Challenges
The next-generation biomanufacturing market faces technical, regulatory, and economic challenges. Regulatory frameworks for advanced therapies remain complex, requiring extensive validation and quality control. Approval timelines increase development costs and time-to-market.
High initial investment in advanced equipment, automation, and digital infrastructure creates financial barriers for small and mid-sized manufacturers. Skilled workforce shortages in bioprocess engineering and data analytics further constrain adoption.
Supply chain dependencies for raw materials such as culture media, single-use components, and specialized enzymes introduce operational risks. Global disruptions can affect production continuity and cost stability.
Data integrity and cybersecurity risks increase with digitalized manufacturing systems. Protecting proprietary processes and patient-related data remains a priority concern for manufacturers.
Market Scope
Segmentation by Type
Single-Use Biomanufacturing Systems
Continuous Bioprocessing Platforms
Modular and Flexible Manufacturing Facilities
Cell and Gene Therapy Manufacturing Systems
Segmentation by Technology
Synthetic Biology
Process Analytical Technology (PAT)
Automation and Robotics
Artificial Intelligence and Digital Twins
Advanced Cell Culture and Fermentation
Segmentation by Application
Biopharmaceutical Production
Vaccine Manufacturing
Cell and Gene Therapies
Industrial Enzymes and Chemicals
Bio-based Materials and Ingredients
Regional Analysis
North America: Largest market with strong biopharmaceutical production and innovation ecosystem
Europe: Focus on sustainable biomanufacturing and regulatory standardization
Asia-Pacific: Fastest-growing region driven by China, India, South Korea, and Singapore
Latin America: Emerging growth supported by public-private partnerships
Middle East & Africa: Early-stage adoption with investments in biotechnology infrastructure
End-User Industries
Pharmaceutical and Biotechnology Companies
Contract Development and Manufacturing Organizations (CDMOs)
Academic and Research Institutions
Industrial Biotechnology Firms
Government and Public Health Agencies
Market Size and Factors Driving Growth
The global next-generation biomanufacturing market size was valued at USD 26.61 billion in 2024 and is expected to reach USD 56.43 billion by 2032, at a CAGR of 9.85% during the forecast period
Key growth drivers include increasing demand for biologics and advanced therapies, particularly monoclonal antibodies, mRNA vaccines, and personalized treatments. Technological innovation reduces production costs and enhances scalability, making advanced therapies more accessible.
Population growth, aging demographics, and rising prevalence of chronic diseases increase demand for complex biologics. Policy support for domestic biomanufacturing capacity, pandemic preparedness, and bioeconomy development strengthens market expansion.
Sustainability initiatives encourage adoption of energy-efficient processes and renewable feedstocks. Emerging regions present strong opportunities due to expanding healthcare infrastructure, growing biotech investments, and government-backed manufacturing programs.
Conclusion
The next-generation biomanufacturing market demonstrates strong growth potential driven by innovation, healthcare demand, and sustainability priorities. Advanced manufacturing platforms enable faster development, improved quality, and greater flexibility across biopharmaceutical and industrial applications.
Innovation remains critical to address regulatory complexity, workforce challenges, and supply chain resilience. Sustainable manufacturing practices and digital transformation will define long-term competitiveness.
Stakeholders including pharmaceutical companies, CDMOs, technology providers, and policymakers stand to benefit from continued investment and global expansion through 2035.
Frequently Asked Questions (FAQ)
1. What is next-generation biomanufacturing?
It refers to advanced biological production systems that use modern technologies to manufacture biologics, vaccines, and bio-based products efficiently and sustainably.
2. What is the current size of the next-generation biomanufacturing market?
The market is valued at approximately USD 22–24 billion as of 2024.
3. What is the expected growth rate of the market?
The market is projected to grow at a CAGR of around 9–10% through 2035.
4. Which applications dominate the market?
Biopharmaceutical production and cell and gene therapy manufacturing are the leading applications.
5. Which region leads the next-generation biomanufacturing market?
North America holds the largest share due to strong biopharma manufacturing capacity and innovation.
6. What are the main challenges in this market?
Regulatory complexity, high capital costs, workforce shortages, and supply chain risks are key challenges.
7. What future opportunities exist in next-generation biomanufacturing?
Opportunities include continuous processing, sustainable biomanufacturing, digital twins, and expansion in emerging biotech markets.
Browse More Reports:
Global Thermocouple Market
Global Transcranial Magnetic Stimulator Market
Global Urine Test Strips Market
Global Vitamin - Mineral Premixes Market
Global Wearable Tracking Devices Market
Global Vinyl Acetate Emulsions Market
Global High Temperature Grease Market
Global Liquid Waste Management Market
Global Plastic Additives Market
Global Well Casing Market
Global Anti-Slip Coatings Market
Global Apheresis Equipment Market
Global Benign Prostatic Hyperplasia Devices Market
Global Commercial Turf Utility Vehicle Market
Global Doy Pouch Packaging Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- [javascript protected email address]






 pallavideshpande
pallavideshpande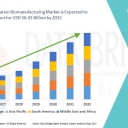
 emmalu222
emmalu222 ameliano111
ameliano111 davidtha
davidtha rawev69
rawev69